- About Us
- Advertise
- Editorial
- Contact Us
- Terms and Conditions
- Privacy Policy
- Do Not Sell My Personal Information
© 2025 MJH Life Sciences™ and Dental Products Report. All rights reserved.
New Product Launches This Week
With the return of in-person live trade shows this week at IDS in Cologne, Germany and DS World 2021 in Las Vegas, Nevada, plenty of new dental product launches were announced this week. Here's just some of the many introductions aimed at improving oral health care.
From whole new implant workflows, to desktop scanners, CAD software, endodontic developments and more, this week's news and new product launches look primed to have a big impact on workflow efficiencies and patient care going forward.

DS PrimeTaper
Three signature workflows to provide dental professionals with a completely new way of practicing implantology and solutions from scan to crown that outperform expectations in efficiency, accuracy, esthetics, longevity and simplicity were introduced today by Dentsply Sirona. Additionally, the company has launched DS PrimeTaper, a self-tapping implant.
The dental manufacturer just announced the comprehensive restage of its implants business, including:
- The Single Tooth Signature Workflow enables customers to do more and better, faster, simpler, freeing up time to see more patients.
- The Partial Multiple Tooth Replacement Signature Workflow solves a signature patient case with three posterior teeth missing to be restored with a 3-unit bridge on 2 implants for more confidence for the clinician and more comfort for the patient.
- The Full Arch Signature Workflow addresses a signature patient case with an edentulous maxilla. The workflow offers a restoration with a fix bridge on 4 implants with immediate temporization.
With a complete ecoystem of digital solutions and products – including well-known brands like Simplant, OSSIX, Axeos, Primescan, Atlantis and MIS – Dentsply Sirona is streamlining implant treatments for practice growth and consistent patient satisfaction.
Dentsply Sirona Implants is now presenting DS PrimeTaper, a self-tapping implant with a tapered design that can be inserted safely and easily. This enables long-term bone stability. The double thread allows the implant to be inserted quickly and safely. A simple drilling protocol, with only a few different drills, supports the workflow.
The company worked closely with the global implant key opinion leader community, and they have already started gaining experience with the product. Their reactions have been very positive:
Dan Butterman, DDS, from Centennial, Colorado, emphasizes the importance of a seamless workflow. “DS PrimeTaper represents the next step in implant dentistry. The digital-driven workflow results in higher accuracy, repeatability of processes, and time savings,” Butterman says. "My patients have better things to do than spending time in my office. Thanks to clearly defined processes and digital solutions, I can offer safer and faster treatment options which my patients appreciate. That's what efficiency is all about."
• Martin Wanendeya, DDS, from the United Kingdom, notes "DS PrimeTaper as a part of an integrated concept is different from what we knew before. In addition to reducing chair time and simplifying collaboration with my laboratory, Primescan for Atlantis suprastructures ensures esthetic predictability and a perfect fit.”
Seamless Concept for Transforming Digital Implant Dentistry
“Dentsply Sirona is about intelligent concepts that work intuitively and improve the predictability of treatment,” says Terri Dolan, DDS, Chief Clinical Officer at Dentsply Sirona. “Our goal is to enable clinicians, including both specialists and general practitioners, to focus on what they do best – creating healthy and beautiful smiles. We are confident that we are building a winning future in the area of implant solutions focused on the needs of our implant customers and their patients. Our connected and seamless concept for transforming digital implant dentistry is a first, essential step in this direction. Our vision for digital implant dentistry will be supported by a global clinical education program focused on end-to-end digital implant workflow solutions to achieve clinic, technical and practice excellence. The extensive curriculum is designed to build clinicians’ digital implant skills according to their level of experience and personal learning preferences.”

Miele Infection Control Line
Dental manufacturer Miele is showcasing its line of sterilizers and instrument cleaners at the IDS this week in Cologne, Germany.
Alongside thermal disinfectors and small sterilizers, air purifiers, washing machines and dishwashers provide protection against viruses and bacteria, and these infection control products are on display at the Miele booth in Cologne from September 22-25.
With their new accessories, Miele thermal disinfectors can be put to universal use, even in the fields of orthodontics and implantology. For the machine-based cleaning and disinfection of multi-function syringes, the company offers matching adapters which guarantee the secure positioning of cannula from a variety of brands in upper and lower baskets.
Additionally, a new insert has been developed which considerably increases the capacity for trays and cartridges–an aspect which simplifies the reprocessing of surgical instruments. All holders can, if required, be inclined to now take 12 trays in the lower basket–twice as many as previously.
If phobic patients have to be treated with laughing gas, the reprocessing of double hoses and masks represents an additional challenge. Early next year Miele will be offering a space-saving solution: A new holder which can hold up to 2 hoses with a capacity that can even be increased to 5. A matching mesh tray is available for reprocessable patients' masks.
Two years after their market launch, the 4 small Miele Cube and Cube X sterilizers offer even greater user convenience by simplifying the reprocessing of instruments in dental practices: these include a new and flexible holder which for the first time accommodates containers and cartridges with a maximum height of 70 mm. Practices which combine the use of a Cube or Cube X unit with a Miele thermal disinfector and hence require considerably more demineralized water now see their needs met fast in the form of Miele full demineralization cartridges.
The company has also developed an app by the name of DataDiary for the wireless transmission of reprocessing data from up to 9 sterilizers. The most recent updated version is available for Android and iOS.
Everywhere in dental practices, Miele machines can reduce the risk of contracting an infection by preventing the spread of viruses and bacteria. Wherever frequent and thorough ventilation is not sufficient, the 3 mobile Miele AirControl air purifiers provide effective assistance: With an air throughput capacity of up to 3,000 cubic meters of air per hour, they are able to replace the entire air in a room 6 times an hour, while at the same time filtering the air as it passes, according to the manufacturer.
A five-stage filtration system which includes a high-performance HEPA H14 particulate filter, captures even the minutest of particles and renders more than 99.995% of all air-borne particles, viruses, bacteria and fungal spores innocuous.
With the company’s System4Dent, a Miele Professional can offer an innovative complete system for the safe and efficient instrument reprocessing in dentists' practices. Based on many years of experience and in close consultation with experts in the field, System4Dent offers a system solution which can be divided into key steps such as cleaning, disinfection, sterilizing, chemical processing, and the highest service quality.

Carbon and Keystone Industries
In what sounds like an ideal partnership, Carbon and Keystone Industries announced that the 2 companies have agreed to develop a flexible removable partial denture resin based on Carbon's patented dual cure materials.
The joint effort will bring about the first use of Carbon's patented dual cure process in a dental application. Carbon is a leading 3D printing technology company, while Keystone Industries, is a leading dental and 3D resin products company.
Providing dental professionals with an innovative process to address the large partially edentulous patient population via the ability to print a flexible partial denture will be the next step in the strategic partnership between the 2 companies that was initiated in 2019. The 2 companies originally partnered to launch KeySplint Soft Clear to Carbon’s 3D printing technology platform, and more recent additions of both KeySplint Hard and KeyGuide. This latest development will allow for the first dental use of Carbon’s patented dual cure process.
"Our dual cure resins meet the needs of a broad range of applications. However, this partnership will result in the first use of Carbon's patented dual cure process in a dental application,” Phil DeSimone, cofounder and Chief Product & Business Development Officer, says. “We're excited to extend our partnership with Keystone to expand denture options for doctors and patients."
This partnership builds on Carbon's industry leadership in developing digital denture solutions that are enabling a new standard of care for edentulous patients. In 2019, Carbon partnered with Dentca to introduce the first FDA-approved 3D printed denture. Carbon was also the first 3D printing technology company to partner with Dentsply Sirona to introduce Lucitone Digital Print, the first high-impact, high esthetic denture base. Nearly a quarter-million denture parts have been printed on the Carbon platform through partnerships with Dentsply Sirona and Dentca.
Keystone Industries has spent decades producing leading denture products and, more recently, ground-breaking 3D printing resins for dental professionals. Keystone’s denture expertise spans a wide array of applications: Diamond D® high-impact denture base acrylic (heat and self cure), KeyMill® high-impact CAD/CAM milling discs for denture bases, ClearMet clear, flexible partial denture resin, Millenium PMMA acrylics, the upcoming high-impact pourable version of Diamond D and in 2022, the KeyDenture 3D printing resins. Using this expansive denture material experience to partner with Carbon’s dual cure 3D process will allow the 2 companies to work towards an innovative breakthrough for partial denture frames.
“We have had a long and productive relationship with Carbon,” notes Keystone Industries dental president Ira Rosenau. “Together, we can leverage several strengths of our companies to bring a very substantial innovation to dental professionals – an efficient way to manufacture custom flexible partial dentures for the many patients who need such a prosthetic device to improve their daily lives."
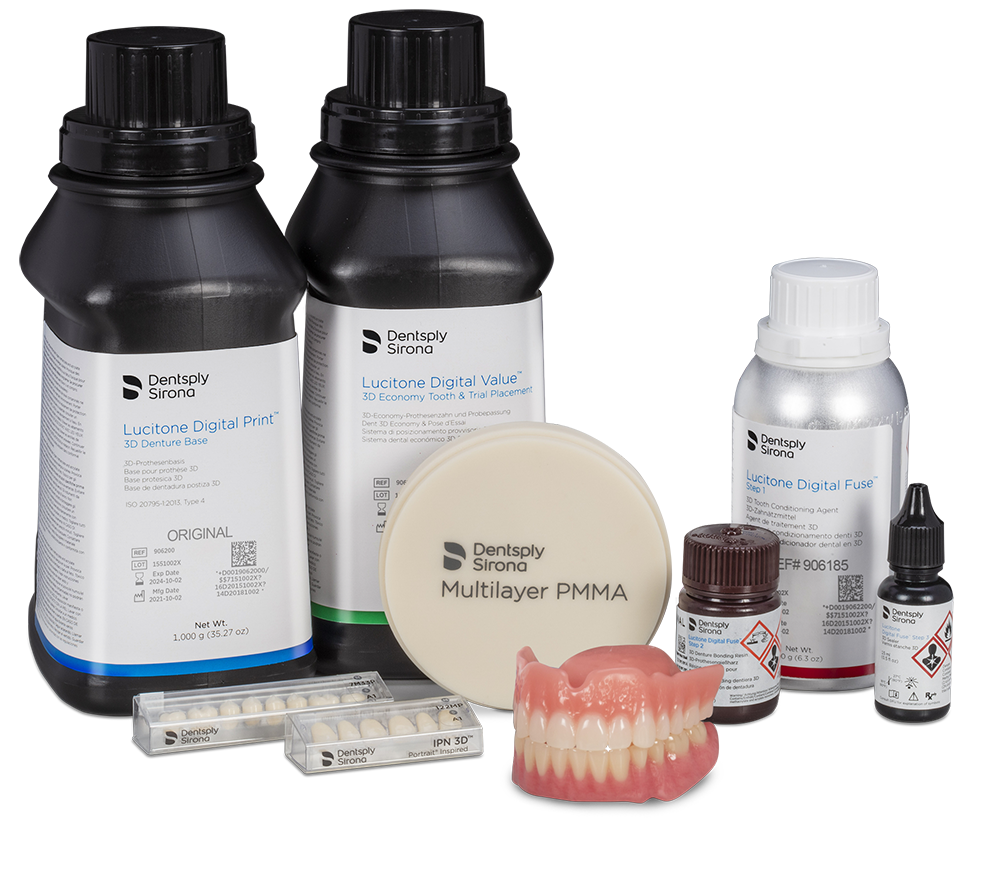
Lucitone Digital Print Denture System
Dentsply Sirona continues to extend the Lucitone Digital Print Denture System with new material indications and planned print platform validations for Asiga and SprintRay, it was announced Thursday while the company is holding DS World 2021 in Las Vegas.
The company announced 4 expansions of the Lucitone Digital Print Denture System, adding momentum to the digital transformation of the dental laboratory industry. Three of these expansions are for the digital production of custom tooth arches and segments, including the addition of Lucitone Digital Value 3D Economy Tooth and Trial Placement resin; DS Multilayer PMMA Discs for milling premium denture teeth; and the planned 2022 release of Lucitone Digital IPN, a printed premium denture tooth material.
Later this year, planned printer validations for the Lucitone Digital Print Denture System will include Asiga Max UV and PRO 4K and SprintRay Pro 95 and Pro 55 series printers. Lucitone Digital Value and DS Multilayer PMMA denture tooth workflows are validated and immediately available for Carbon M-Series printers.
These important updates provide dental laboratories greater flexibility to fully digitize their denture production with the superior strength Lucitone Digital Print Denture System. The forthcoming validation of Asiga and SprintRay printers will enable more labs to benefit from the enhanced capacity, throughput and simplicity of Dentsply Sirona printed dentures, delivering repeatable and precise results.
“This collaboration with Dentsply Sirona continues Asiga’s open-system vision to bring best-in-class, highly accurate, highly affordable printing technology to the dental industry,” says Justin Elsey, founder and managing director of Asiga. “Together, we are opening access to dental labs and their patients to a superior combination of printed denture materials and printer accuracy and consistency.”
The MAX series from Asiga features repeatable and precise desktop 3D printers, while the company’s PRO 4K is a larege format floor-standing 3D printer.
“At SprintRay, we strive to build products that work together to create opportunity for collaboration, innovation and, most importantly, outstanding clinical results,” says Amir Mansouri, CEO and Co-Founder. “Our cooperation with Dentsply Sirona embodies this vision by bringing together high-quality products to deliver first-class service to patients all the while empowering improved efficiency and production for dental practices and labs everywhere.”
“Until the introduction of the Lucitone Digital Print Denture System, printed dentures were not a viable option for dental labs,” shares Julie Mroziak, Vice President, Dentsply Sirona Digital Lab and Preventive Solutions. “Now, with the planned validation of Asiga and SprintRay printers for Lucitone Digital Print Denture, and more material options, labs can begin transitioning from analog denture procedures to the more efficient, digitally printed denture workflows. The digital acceleration of the removable lab industry is truly exciting, and a win-win for the lab, dentist and patient experience.”
With the versatile Dentsply Sirona Multilayer PMMA 98mm disc range, dental labs now can mill custom tooth arches or segments to digitally manage more challenging removable cases, including reduced vertical and Class II and Class III occlusions. The disc product line provides comprehensive shade choices, 17 in total, three-disc thicknesses, and delivers premium tooth esthetics.
Additionally, the dual-use Lucitone Digital Value 3D Economy Tooth & Trial Placement resin allows for printing try-in appliances and economy tooth arches and segments. This ready-to-print tooth material balances economic durability and esthetics. The material is available in 6 shades and suitable for all clinical classifications of full-arch denture cases.
The planned 2022 release of Lucitone Digital IPN will be Dentsply Sirona’s first printed premium denture tooth solution. This new material is expected to have exceptional wear characteristics and include 16 shades plus bleach options. The Lucitone Digital IPN material is designed to remove all barriers for dental laboratories’ migration to digital denture workflows.
The clinical and laboratory workflows are simple and easy for rapid integration. With these anticipated printer validations and material launches, laboratories of all sizes can better meet increasing volume demands, achieve greater profitability, and provide patients with an experience and denture that will make them smile in partnership with their dentist.

Gumline Cleaner
Some commercially available products consumers have been using to fight gum disease are outdated and ineffective. Evidence of this comes from the fact that nearly half of Americans over 30 have some form of gum disease, and there has not been enough innovation in products that are designed to fight it – until now, according to the manufacturer bäz.
Jean-Jacques Elbaz, DDS, a Beverly Hills-based periodontist and expert on the Dental Board of California, developed a game-changing product: the Gumline Cleaner. By tracing the gum line, users can manually remove sticky plaque that builds in these areas by using friction.
Additionally, the soft tip massages gum tissue – promoting blood flow – and the bristles provide abrasion for effective biofilm removal. Not an interdental pick, the Gumline Cleaner focuses on the gingival margin. Its ergonomic handle allows the user to clean distal and hard-to-reach areas that toothbrushes can’t reach.
The new product is especially effective for patients who have traditional braces or aligner trays, recessions, dental implants, crowns & bridges, crowding, gingival inflammation, and more. Patients can find it online or at select dental practices. There are hundreds of toothbrushes, dozens of flossing and interdental products, lots of mouth rinses, but now there’s Gumline Cleaner, designed to provide better protection against plaque buildup around the gum line than what is achieved by brushing and rinsing with traditional products.

Ceramill Map 600+
Quicker turnaround times are just 1 of the many features included with the fully automatic Ceramill Map 600+, Amann Girrbach's new scanner flagship for open articulator scanning that was announced on Thursday. The scanner is designed to deliver outstanding precision for perfect restorations and optimally support dental technicians in their work.
This new high-performance scanner heralds the advent of Industry 4.0 in the laboratory. The intelligent software algorithm automatically assigns the upper and lower jaws, thereby eliminating the vestibular scan and thus up to 30 percent of the manual steps in the laboratory. Due to its integrated universal carrier plate for all common types of articulators, the Map 600+ saves time-consuming plate changes and the intelligent scan height control automatically moves the object to be scanned into the best possible scanning area.
Amann Girrbach has equipped the Map 600+ with an Ultra HD camera. The highly sensitive industrial 3D sensor with Blue Light technology is designed to ensure outstanding depth of field and a scanning accuracy of 4 micrometers. Making optimal use of the advantages of digitization and a seamless workflow requires the model situation from the real articulator to be converted into a data set with maximum precision.
The scanner’s new, more efficient calculation algorithm also reduces the matching time by up to 35 percent, depending on the indication. This reduces the active waiting time of a scanning process by up to 25 seconds. Depending on the indication, the Ceramill Map 600+ therefore provides time savings of between 15 and 38 percent, according to Amann Girrbach.
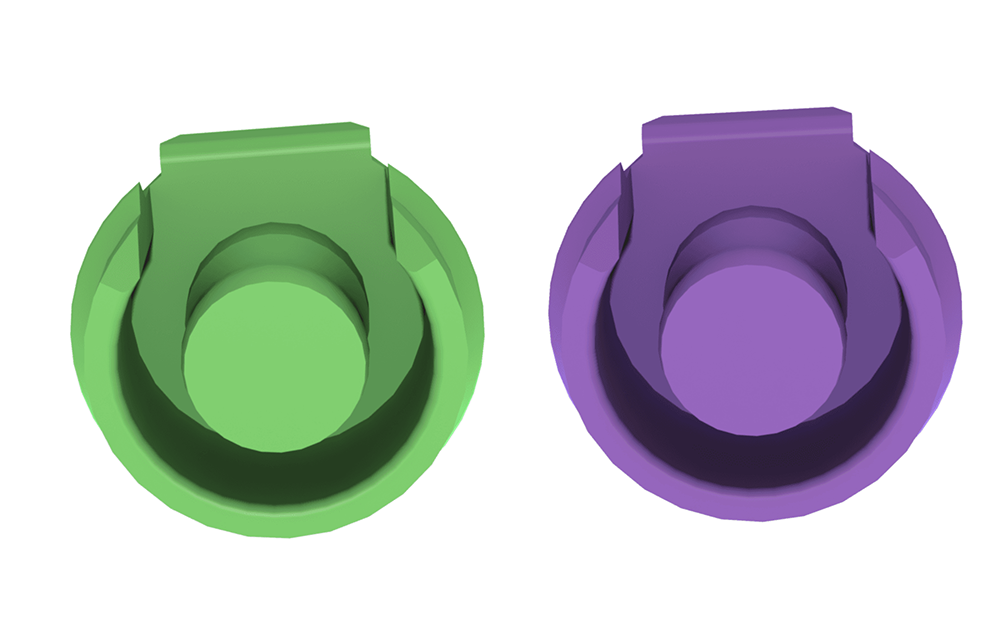
ERA RV Male Attachment / Green and Purple
Sterngold’s original ERA® overdenture partial denture attachment system has been the choice of many dentists since its development in 1991—remains the standard in the industry today—and now the company has released the ERA RV Male Attachment, new green and purple extra oversized males, new options for long-time ERA patients.
Before the introduction of the ERA concept, patients and dental professionals alike were frequently frustrated by high costs from other options, complexity (other options could be difficult to fabricate, implement or maintain) and most important, resiliency. In the past, some options have been unreliable for long-term patient use.
ERA attachments consist of a metal female component, which is intraorally fixed, and a replaceable, high density nylon male anchored in the denture base. Smaller and more resilient, the ERA attachments are designed to deliver much greater overall performance.
Directing more force away from the implants or attachment side, the ERA allows the prosthesis to remain tissue supported, generating less wear to both the attachment as well as the insert. This means greater comfort, stability, and ease of use for the patient, according to Sterngold.
As clinical studies have proven the longevity and reliability of these attachments over several decades, Sterngold now offers green and purple retentive inserts for the ERA® RV partial denture attachment system.
ERA Male Attachments — Levels of Retention
Every attachment procedure begins with the black processing insert. This insert has a built-in spacer so it sits rigidly on top of the female. When you’re ready to remove and replace the black male insert, you have a multitude of options, each one increasing in retention. For many years, your ERA® male attachment insert options were limited to the following:
- White (least retentive)
- Orange
- Blue
- Gray
- Yellow
- Red (most retentive)
Now Sterngold is offering even greater retention with these 2 additional ERA male attachment options:
- Green
- Purple
There are 4 types of ERA males: partial denture — ERA RV, Micro ERA, ERA Overdenture, and Micro ERA Overdenture. In any case, all ERA males are mechanically anchored in the denture base, providing both vertical resiliency as well as universal hinge movement.
To implement an ERA RV attachment, you can purchase all the materials you’ll need in the company’s Stern ERA RV Starter Kit. This kit includes:
- 2 attachments
- 2 metal jackets
- 2 processing jigs
- 1 core cutter bur
- 1 seating tool
- 1 paralleling mandrel
Additionally, the process of removing and replacing ERA males is simple. Worn out males can be removed with a specially designed bur and the new ones simply snap into a metal jacket that’s been permanently processed into the denture. Simply follow these steps:
- Use the core cutter trephine bur to remove the center post of the male.
- Pop the remnant of the male out with the ERA® extraction tool.
- Put a new male on the seating tool.
- Snap the new male into the metal jacket or denture acrylic.
Resiliency, dependability, and budget-friendly, the ERA attachment system is a dependable choice for removable partial denture and overdenture treatments. The addition of new retention inserts only expands the number of patients who can now be considered for this proven treatment method.



